IMPORTANT SAFETY INFORMATION
• VASCEPA is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to VASCEPA or any of its components
• VASCEPA was associated with an increased risk (3% vs 2%) of atrial fibrillation or atrial flutter requiring hospitalization in a double-blind, placebo-controlled trial. The incidence of atrial fibrillation was greater in patients with a previous history of atrial fibrillation or atrial flutter
• It is not known whether patients with allergies to fish and/or shellfish are at an increased risk of an allergic reaction to VASCEPA. Patients with such allergies should discontinue VASCEPA if any reactions occur
INDICATIONS AND LIMITATIONS OF USE
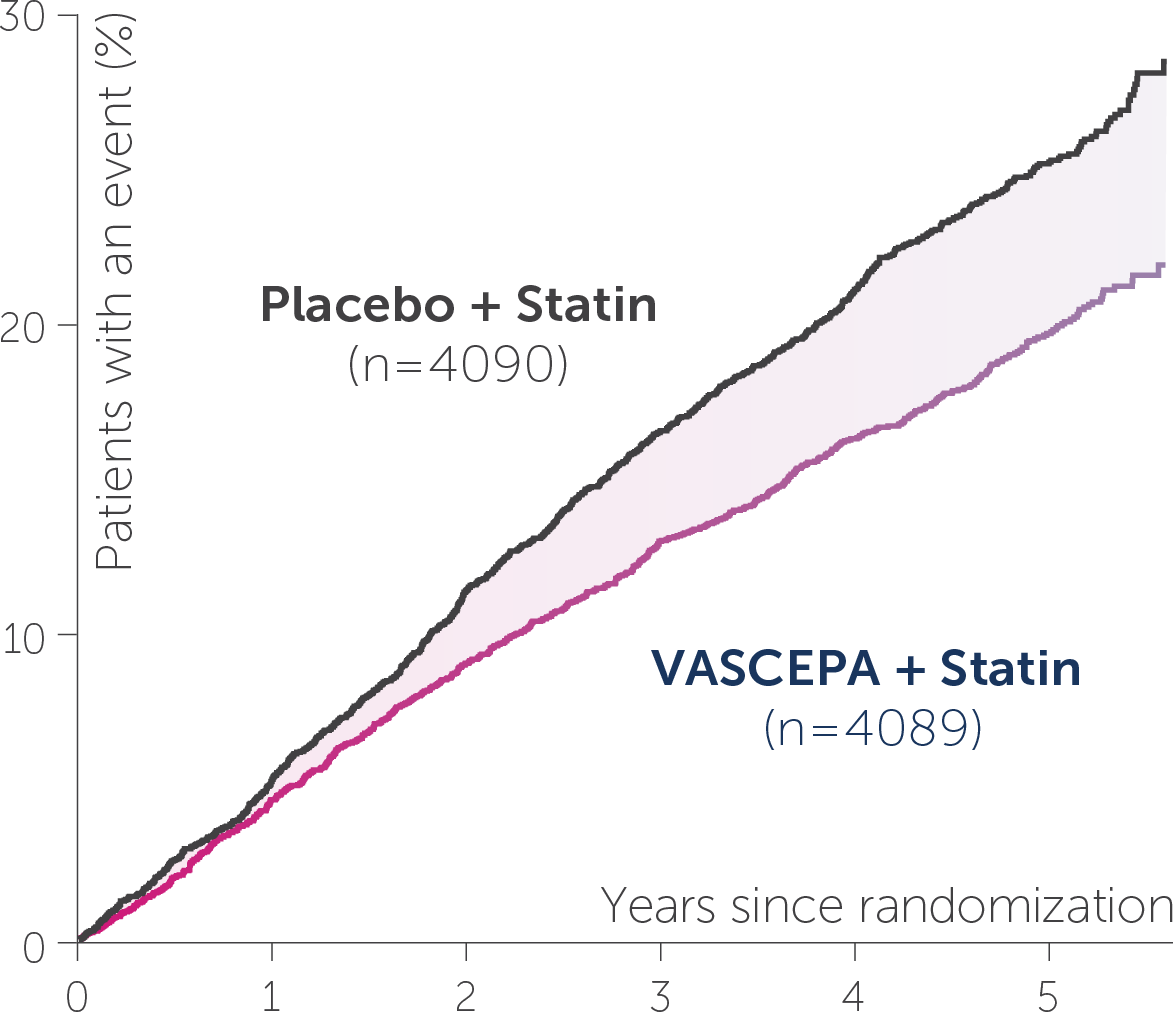
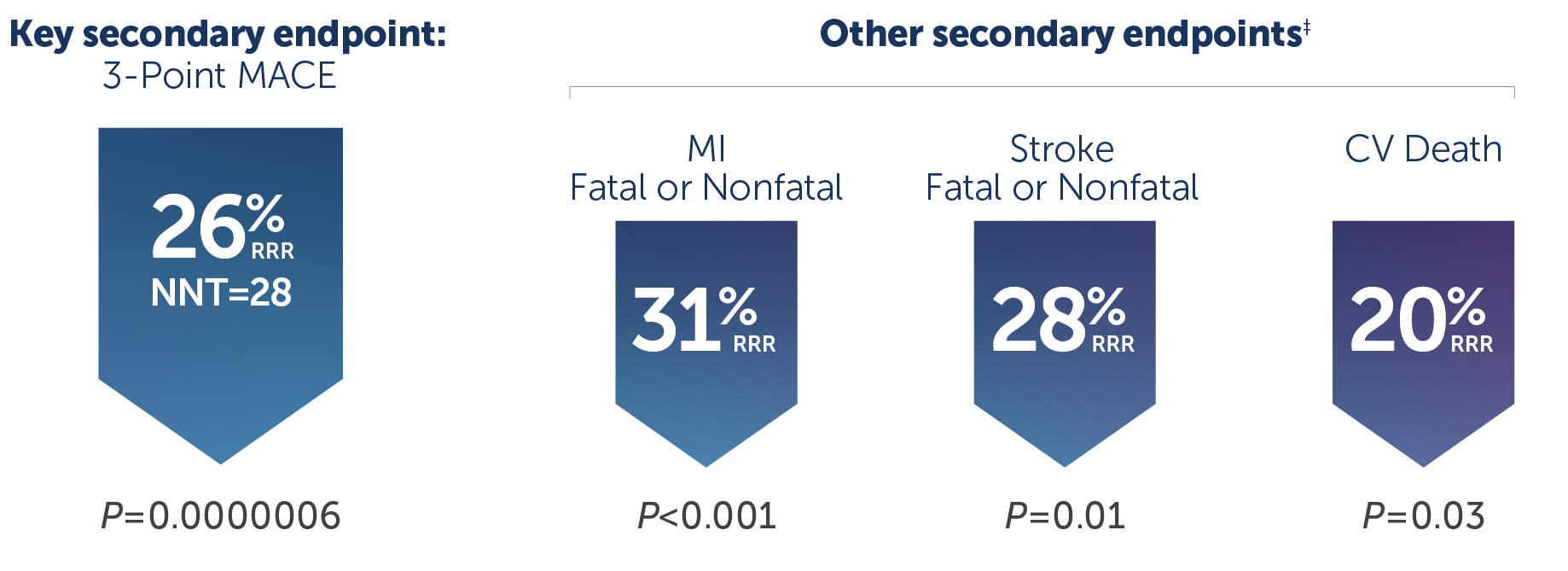
• VASCEPA® (icosapent ethyl) is indicated as an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥150 mg/dL) and established cardiovascular disease or diabetes mellitus and 2 or more additional risk factors for cardiovascular disease
• VASCEPA is indicated as an adjunct to diet to reduce TG levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia
The effect of VASCEPA on the risk for pancreatitis in patients with severe hypertriglyceridemia has not been determined.